

(Remember to multiply the coefficient 3 by each of the subscripts to get the total per atom.) H 2 O has 2 atoms of hydrogen and 1 atom of oxygen.Ĭ a C l 2 has 1 atom of calcium and 2 atoms of chlorine.ģ C 6 H 12 O 6 has 18 atoms of carbon, 36 atoms of hydrogen, and 18 atoms of oxygen. If there is no number, it represents a 1. So, how do you know how many atoms are present in a chemical? The rule is to take the coefficient (the number in front of the chemical formula) multiplied by the subscript (the number to the lower right of the element symbols) for each atom in the formula.
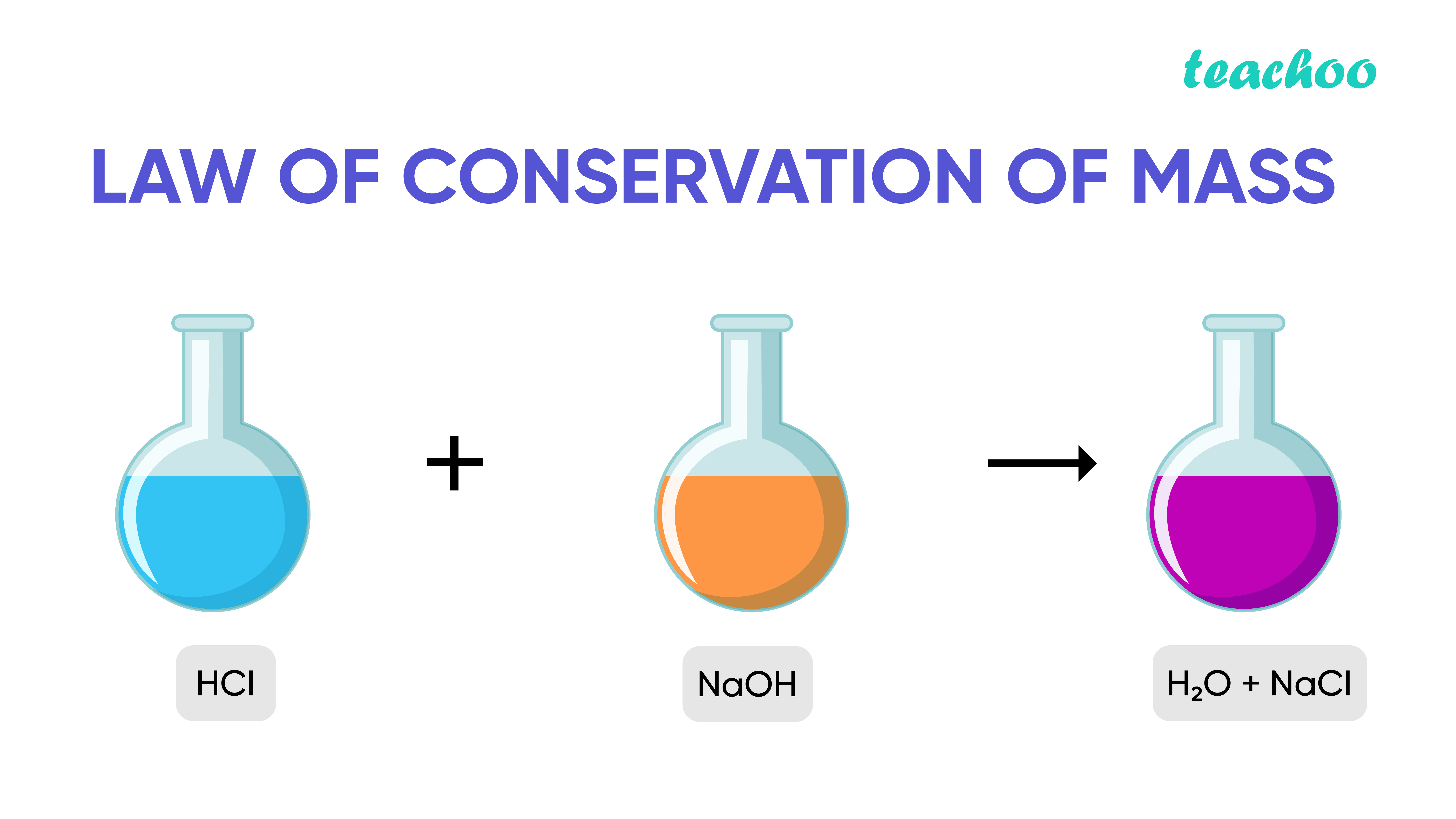
Atoms can’t just disappear! They might be in a different arrangement within new molecules that have formed, but there is the same number overall. If there are 5 sulfur atoms before a reaction, there will also be 5 sulfur atoms after the reaction. As you just saw in the videos, the number of each type of atom is the same before and after a reaction.


 0 kommentar(er)
0 kommentar(er)
